Seznamy Model Of Atom By Rutherford
Seznamy Model Of Atom By Rutherford. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Originally, an atom was thought to be the smallest unit in existence.
Nejchladnější Rutherford Atomic Model Electrical4u
To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.
The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. He called this region of the atoms a nucleus. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: He called this region of the atoms a nucleus. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume... Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.
The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: Originally, an atom was thought to be the smallest unit in existence. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.

According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Originally, an atom was thought to be the smallest unit in existence. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … He called this region of the atoms a nucleus. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.

To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at ….. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Originally, an atom was thought to be the smallest unit in existence. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd... According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom... . According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. He called this region of the atoms a nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford... The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.

The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:

The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... He called this region of the atoms a nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Originally, an atom was thought to be the smallest unit in existence. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:.. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:. He called this region of the atoms a nucleus. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume... According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume... Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. He called this region of the atoms a nucleus. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …
Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Originally, an atom was thought to be the smallest unit in existence... Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:

He called this region of the atoms a nucleus.. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom... According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.. He called this region of the atoms a nucleus.

The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... He called this region of the atoms a nucleus.. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.

The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... . According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. He called this region of the atoms a nucleus. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at ….. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Originally, an atom was thought to be the smallest unit in existence. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists... Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd... Originally, an atom was thought to be the smallest unit in existence. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. He called this region of the atoms a nucleus. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. He called this region of the atoms a nucleus. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Originally, an atom was thought to be the smallest unit in existence. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called …

The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The major part of the alpha particles which was struck to the gold foil would pass through it without deflections... He called this region of the atoms a nucleus.

To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. He called this region of the atoms a nucleus. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.

Originally, an atom was thought to be the smallest unit in existence. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. He called this region of the atoms a nucleus. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Originally, an atom was thought to be the smallest unit in existence. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Originally, an atom was thought to be the smallest unit in existence.

Originally, an atom was thought to be the smallest unit in existence. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. He called this region of the atoms a nucleus. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.

The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford... Originally, an atom was thought to be the smallest unit in existence. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: He called this region of the atoms a nucleus. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists... He called this region of the atoms a nucleus.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Originally, an atom was thought to be the smallest unit in existence. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. He called this region of the atoms a nucleus. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume... According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd... According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. He called this region of the atoms a nucleus. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. Originally, an atom was thought to be the smallest unit in existence.

Originally, an atom was thought to be the smallest unit in existence. Originally, an atom was thought to be the smallest unit in existence. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom... The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom... The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. He called this region of the atoms a nucleus. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Originally, an atom was thought to be the smallest unit in existence. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Originally, an atom was thought to be the smallest unit in existence. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford... To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

He called this region of the atoms a nucleus... Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Originally, an atom was thought to be the smallest unit in existence. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. He called this region of the atoms a nucleus... According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford... He called this region of the atoms a nucleus. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. He called this region of the atoms a nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Originally, an atom was thought to be the smallest unit in existence. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Originally, an atom was thought to be the smallest unit in existence. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. He called this region of the atoms a nucleus. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen... According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.. Originally, an atom was thought to be the smallest unit in existence.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … He called this region of the atoms a nucleus. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Originally, an atom was thought to be the smallest unit in existence. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd... He called this region of the atoms a nucleus.

The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. He called this region of the atoms a nucleus. Originally, an atom was thought to be the smallest unit in existence. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen... The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. He called this region of the atoms a nucleus. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Originally, an atom was thought to be the smallest unit in existence. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.

The major part of the alpha particles which was struck to the gold foil would pass through it without deflections... The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … He called this region of the atoms a nucleus. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.

The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Originally, an atom was thought to be the smallest unit in existence. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. He called this region of the atoms a nucleus. Originally, an atom was thought to be the smallest unit in existence. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The major part of the alpha particles which was struck to the gold foil would pass through it without deflections... To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Originally, an atom was thought to be the smallest unit in existence. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at …

Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.

The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Originally, an atom was thought to be the smallest unit in existence. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called …

According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. . According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

He called this region of the atoms a nucleus. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.

Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:.. He called this region of the atoms a nucleus. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... Originally, an atom was thought to be the smallest unit in existence.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.. . Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen... Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.. Originally, an atom was thought to be the smallest unit in existence.

The major part of the alpha particles which was struck to the gold foil would pass through it without deflections... Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … He called this region of the atoms a nucleus. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Originally, an atom was thought to be the smallest unit in existence.. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:

Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Originally, an atom was thought to be the smallest unit in existence. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists... The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.

According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. He called this region of the atoms a nucleus. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.

He called this region of the atoms a nucleus... To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: Originally, an atom was thought to be the smallest unit in existence. He called this region of the atoms a nucleus. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

He called this region of the atoms a nucleus. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.
Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen... The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Originally, an atom was thought to be the smallest unit in existence. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.. Originally, an atom was thought to be the smallest unit in existence.

The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen... According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called …

The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called …. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Originally, an atom was thought to be the smallest unit in existence.. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

Originally, an atom was thought to be the smallest unit in existence.. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Originally, an atom was thought to be the smallest unit in existence. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. He called this region of the atoms a nucleus. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called …. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.

The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.

The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:

Originally, an atom was thought to be the smallest unit in existence. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.
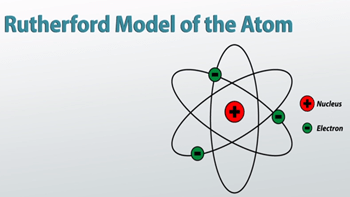
Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom.. Originally, an atom was thought to be the smallest unit in existence. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd.. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford.

The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists.. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen.

Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd... Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. Originally, an atom was thought to be the smallest unit in existence. He called this region of the atoms a nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called ….. The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

Originally, an atom was thought to be the smallest unit in existence... The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. Originally, an atom was thought to be the smallest unit in existence. To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … The major part of the alpha particles which was struck to the gold foil would pass through it without deflections. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Rutherford's nuclear model also proposed that the negatively charged electrons encircle the nucleus of an atom. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford.

Originally, an atom was thought to be the smallest unit in existence. Het atoommodel van rutherford verving het atoommodel van thomson maar het model verklaarde nog niet hoe de atoomkern en de elektronenwolk uit deze deeltjes was opgebouwd. Originally, an atom was thought to be the smallest unit in existence. Rutherford stelde dat het atoom bestond uit een kern met daarin een aantal zware, subatomaire deeltjes met de centrale lading, en daarrond de elektronen. He called this region of the atoms a nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called … To know more about rutherford's alpha scattering experiment, limitations of rutherford's model of atom here at … According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford. According to the accepted atomic model, in which an atom's mass and charge are uniformly distributed throughout the atom, the scientists. According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.

The major contribution in knowing the relative positions of electrons and protons in an atom was by ernest rutherford... According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow: The rutherford model of the atom is a model of the atom devised by the british physicist ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called …. Based on the experiment that he performed, rutherford concluded some points regarding the structure of atom as follow:

According to rutherford's atomic model, the positively charged particles and most of the atom's mass was concentrated in a minimal volume.. The major part of the alpha particles which was struck to the gold foil would pass through it without deflections.
